
About the DSCSA Traceability Report
The DSCSA Traceability Report, accessible under REPORTS, complies with the Drug Supply Chain Security Act (DSCSA), which aims to improve patient safety by identifying and tracing drugs at the package level as they move through the supply chain.

Running the Report
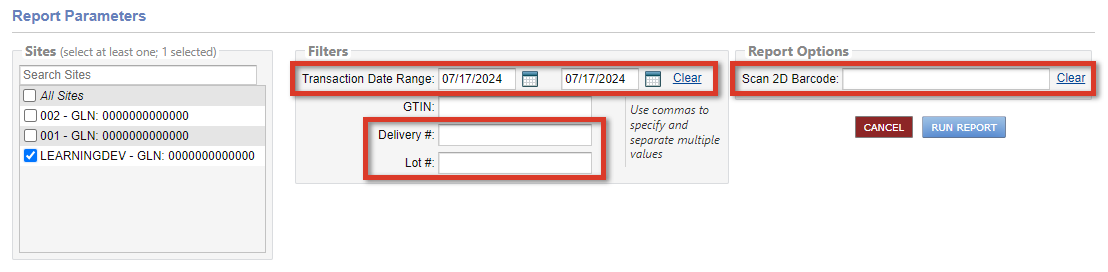
The DSCSA Traceability Report displays inventory provenance from McKesson and can be run for up to ten Lynx sites at a time.
For multisite practices, use the Sites filter to select which sites to include in the report results.
When running the report, a Transaction Date Range of up to 90 days must be entered, unless a Delivery # and/or Lot # is provided.
Practices using barcode scanners with Lynx can click in the Scan 2D Barcode field and scan an item to locate the DSCSA information for the item.

Report Results
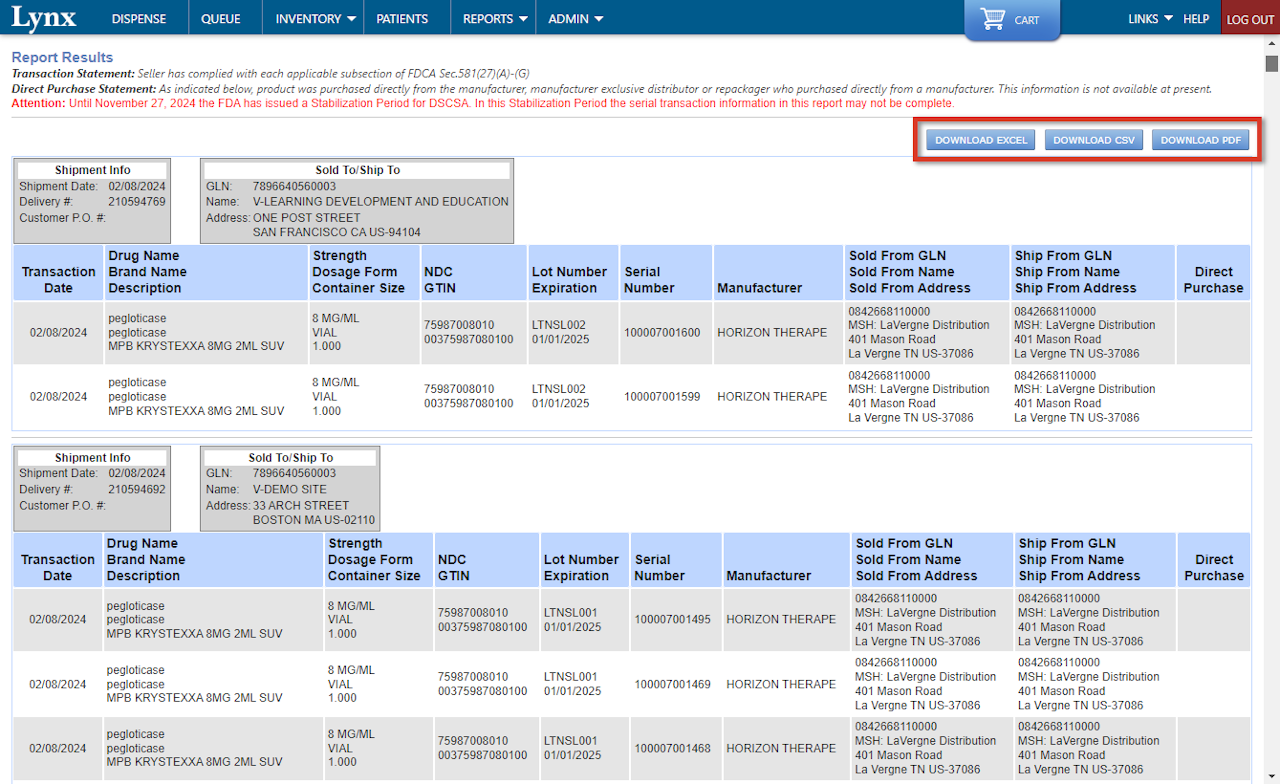
The report is organized by Sold To/Ship To site and displays information about purchases placed for each site.
Each line of the report includes information about the purchase transaction, such as the Transaction Date and the item purchased, as well as the Lot Number, Expiration, Serial Number, Manufacturer, distribution center that fulfilled the order, and whether the item was a Direct Purchase (drop-shipped).
For convenience, the DSCSA Traceability Report can be exported as a EXCEL file, comma separated values (CSV), and as a PDF.
